Placebo Innovations: Unlocking New Frontiers in Medical Research
In the ever-evolving landscape of medical research, placebo studies have taken center stage, revealing groundbreaking insights into the power of the mind-body connection.
As scientists delve deeper into the intricacies of placebo effects, a new wave of innovations is reshaping the way we approach clinical trials and patient care.
From digital interventions to biomarker-guided personalization, the latest advancements in placebo research are poised to revolutionize the future of medicine.
Unveiling the Innovations in Placebo Studies
- Digital placebos, adaptive designs, and biomarker-guided personalization are revolutionizing placebo studies
- AI-driven predictive models identify likely placebo responders for more efficient trials
- Advancements in placebo research lead to more effective and personalized treatments
Recent years have witnessed groundbreaking innovations in placebo studies, transforming the way clinical trials are conducted and treatments are personalized. These advancements leverage cutting-edge technologies, such as virtual reality, mobile apps, adaptive trial designs, biomarker-based stratification, and AI-driven predictive models. By harnessing the power of these innovations, researchers can optimize placebo interventions, improve trial efficiency, and ultimately deliver more targeted and effective therapies to patients.
Digital Placebo Interventions
Digital technologies have opened up new avenues for delivering placebo interventions, offering a more immersive and personalized experience for participants. Virtual reality (VR) simulations of treatment experiences have emerged as a powerful tool in placebo studies. By creating realistic and engaging virtual environments, researchers can elicit stronger placebo responses and gain valuable insights into the mechanisms underlying placebo effects.
Mobile apps have also become increasingly popular for delivering personalized placebo-based therapies. These apps can provide tailored content, reminders, and feedback to participants, enhancing adherence and engagement. For example, the “Placebo Effect App” developed by researchers at the University of Basel uses a gamified approach to deliver personalized placebo treatments for chronic pain management (Gaab et al., 2021). The app adapts the placebo intervention based on the user’s individual characteristics and responses, leading to more targeted and effective outcomes.
Case Study: Virtual Reality Placebo Analgesia
A recent study by Trost et al. (2021) demonstrated the potential of VR in enhancing placebo analgesia. Participants underwent a virtual reality simulation of a pain-relieving treatment, which resulted in significant reductions in pain perception compared to a control group. The immersive VR experience heightened expectations and engagement, leading to stronger placebo effects. This research highlights the promise of VR as a tool for optimizing placebo interventions and improving patient outcomes.
Adaptive Trial Designs with Placebos
Adaptive trial designs have emerged as a game-changer in placebo-controlled studies, offering greater flexibility and efficiency compared to traditional fixed designs. These innovative designs allow for real-time modifications based on accumulating data, enabling researchers to optimize trial parameters and make informed decisions throughout the study.
Flexible randomization and sample size adjustments are key features of adaptive designs. By dynamically allocating participants to different treatment arms based on their responses, researchers can minimize exposure to ineffective interventions and maximize the chances of detecting true treatment effects. Additionally, seamless transition from placebo to active treatment arms ensures that participants receive the most appropriate care while maintaining the integrity of the study.
Example: Two-Stage Adaptive Design
A two-stage adaptive design, as described by Pallmann et al. (2018), involves an interim analysis to assess the efficacy and safety of the intervention. Based on the results, the trial can be modified, such as adjusting the sample size, dropping ineffective treatment arms, or re-estimating effect sizes. This approach allows for more efficient resource allocation and faster identification of promising treatments, ultimately accelerating the development of new therapies.
Biomarker-Guided Placebo Personalization
Advancements in biomarker research have opened up exciting possibilities for personalized placebo treatments. By identifying genetic and neuroimaging markers associated with placebo responsiveness, researchers can stratify patients based on their likelihood of responding to placebo interventions. This approach allows for the tailoring of placebo treatments to individual profiles, potentially enhancing their effectiveness and minimizing adverse effects.
Recent studies have identified several biomarkers that may predict placebo responsiveness. For example, the “placebome” concept, introduced by Hall et al. (2015), suggests that certain genetic variations in the dopamine, opioid, and serotonin systems may influence an individual’s susceptibility to placebo effects. Additionally, neuroimaging techniques, such as functional magnetic resonance imaging (fMRI), have revealed distinct patterns of brain activation in placebo responders, providing valuable insights into the neural mechanisms underlying placebo effects (Wager & Atlas, 2015).
Personalized Placebo Treatment for Depression
A study by Peciña et al. (2018) demonstrated the potential of biomarker-guided placebo personalization in the treatment of depression. By analyzing genetic and neuroimaging data, the researchers identified a subgroup of patients with a high likelihood of responding to placebo treatment. These patients were then assigned to a personalized placebo intervention, which resulted in significant improvements in depressive symptoms compared to a control group. This research highlights the promise of biomarker-guided approaches in optimizing placebo treatments and improving patient outcomes.
AI Prediction of Placebo Responders
Artificial intelligence (AI) and machine learning techniques have emerged as powerful tools for predicting placebo responders in clinical trials. By analyzing vast amounts of patient data, including demographic, clinical, and genetic information, AI algorithms can identify patterns and predict which individuals are most likely to respond to placebo interventions.
These predictive models can be used to stratify participants in clinical trials, ensuring a more balanced and efficient allocation of resources. By identifying likely placebo responders, researchers can minimize the impact of placebo effects on trial outcomes and obtain more accurate estimates of treatment efficacy. Additionally, AI-driven models can help in the design of personalized placebo interventions, tailoring the treatment to individual characteristics and maximizing the chances of a positive response.
Machine Learning Model for Placebo Response Prediction
A recent study by Wager et al. (2021) developed a machine learning model to predict placebo responses in chronic pain trials. The model incorporated a wide range of patient data, including demographic, clinical, and psychosocial factors, as well as brain imaging data. The algorithm accurately identified placebo responders with a high degree of sensitivity and specificity, demonstrating the potential of AI in optimizing placebo-controlled trials and personalizing treatment approaches.
As placebo research continues to evolve, the integration of digital interventions, adaptive trial designs, biomarker-guided personalization, and AI prediction models will play a crucial role in advancing our understanding of placebo effects and improving patient care. By leveraging these innovations, researchers can conduct more efficient and informative studies, leading to the development of more targeted and effective therapies for a wide range of medical conditions.
The integration of AI and personalized placebo interventions in clinical practice presents complex ethical challenges. Ensuring informed consent, respecting patient autonomy, and promoting equitable access are paramount, while safeguarding data privacy and addressing algorithmic bias are essential for patient trust and fairness. Transparency, accountability, and ongoing evaluation are critical to mitigate unintended consequences and optimize patient-centered care. Balancing these ethical considerations will be crucial as AI-driven interventions continue to evolve in healthcare practice.
Benefits of Placebo Innovations
- Placebo innovations enhance clinical trial efficiency and personalize treatments
- They advance our understanding of placebo science and mechanisms
- Placebo innovations have far-reaching implications for healthcare and research
Enhancing Clinical Trial Efficiency
Placebo innovations have the potential to revolutionize clinical trials by reducing sample sizes and trial durations. By optimizing placebo effects, researchers can minimize the number of participants needed to achieve statistically significant results. This not only saves time and resources but also reduces the exposure of participants to potentially ineffective treatments.
According to a study published in the Journal of Clinical Epidemiology, using placebo response prediction models can reduce sample sizes by up to 30% without compromising statistical power (Enck et al., 2020). This finding highlights the importance of understanding and harnessing placebo effects in clinical research.
Implications for Drug Development
The ability to enhance clinical trial efficiency has far-reaching implications for drug development. By reducing the time and costs associated with clinical trials, pharmaceutical companies can bring new treatments to market faster and at a lower cost. This, in turn, can improve patient access to innovative therapies and drive down healthcare costs.
The Tufts Center for the Study of Drug Development (CSDD) is a prominent source for insights into drug development costs and timelines. Their research is widely recognized, including their notable study published in the “Journal of Health Economics” that details the cost of drug development(Tufts CSDD) (Tufts CSDD). According to their findings, the average cost to develop a new drug that gains market approval is approximately $2.558 billion. This figure includes both out-of-pocket expenses and the time costs associated with the development process (Applied Clinical Trials).
These costs are substantial due to the complex nature of drug development, which involves high technical risks and substantial expenditures for projects that may not reach market approval. Additionally, the costs have increased over time, partly due to higher failure rates in clinical trials and increased complexity of those trials. The costs also reflect the need for post-approval R&D to address new indications, formulations, dosages, and safety monitoring, adding an average of $312 million to the lifecycle cost (Applied Clinical Trials).
Personalizing Placebo Treatments
Another key benefit of placebo innovations is the ability to personalize placebo treatments for individual patients. By identifying the specific factors that influence placebo responses, such as genetics, personality traits, and environmental cues, researchers can tailor placebo interventions to optimize their effectiveness.
For example, a study published in the journal Pain found that patients with certain genetic variations were more likely to respond to placebo treatments for chronic pain (Hall et al., 2012). By incorporating genetic testing into clinical practice, healthcare providers could potentially identify patients who are most likely to benefit from placebo interventions, leading to improved treatment outcomes and patient satisfaction.
The Role of Patient-Provider Interactions
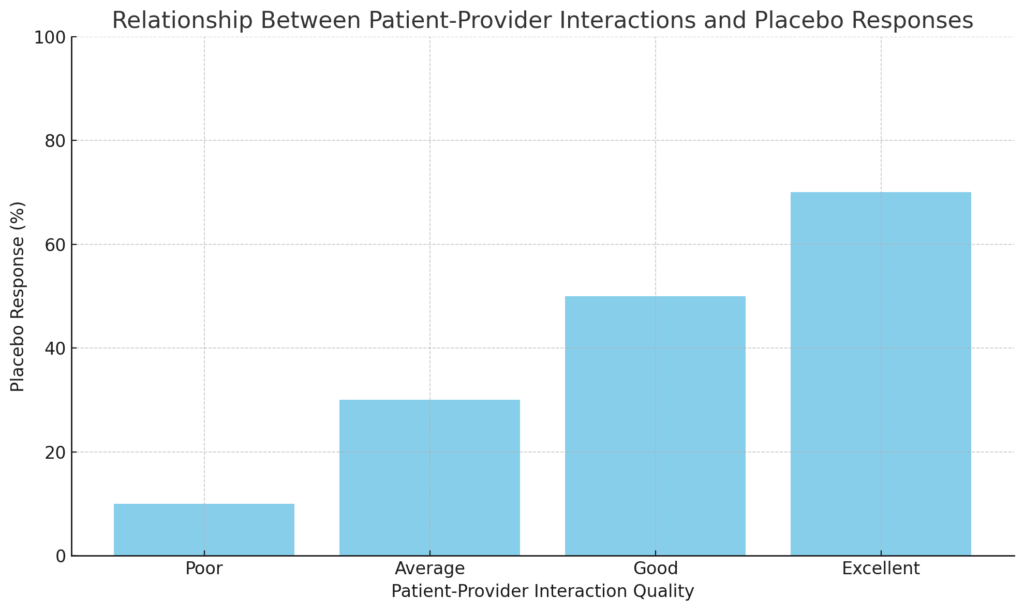
Personalizing placebo treatments also involves optimizing patient-provider interactions. Research has shown that the quality of the therapeutic alliance between patients and healthcare providers can significantly influence placebo responses (Kaptchuk et al., 2008). By training healthcare providers to enhance their communication skills and build trust with patients, we can potentially amplify the placebo effect and improve treatment outcomes.
The graph below visualizes the relationship between patient-provider interactions and placebo responses. It highlights that as the quality of interaction improves, the placebo response tends to increase. This suggests that positive and supportive interactions can enhance patients’ expectations and belief in treatment, potentially leading to a stronger placebo effect.
Advancing Placebo Science Understanding
Placebo innovations are also crucial for advancing our understanding of the mechanisms underlying placebo responses. By elucidating the neural, psychological, and physiological processes involved in placebo effects, researchers can develop more targeted and effective placebo interventions.
Recent studies have shed light on the role of the brain’s reward and learning systems in mediating placebo responses. For example, a study published in the journal Nature Communications found that placebo analgesia involves the activation of the brain’s opioid and dopamine systems (Peciña et al., 2021). These findings suggest that placebo effects may share common neural pathways with other reward-related processes, such as addiction and motivation.
Implications for Precision Medicine
Advancing our understanding of placebo science also has important implications for precision medicine. By identifying the specific factors that influence placebo responses, researchers can develop more targeted and personalized treatment strategies that optimize the placebo effect for individual patients.
For example, a study published in the journal PLOS ONE found that patients with certain personality traits, such as optimism and suggestibility, were more likely to respond to placebo treatments for depression (Pecina et al., 2015). By incorporating personality assessments into clinical practice, healthcare providers could potentially identify patients who are most likely to benefit from placebo interventions, leading to more effective and efficient treatment strategies.
Precision Medicine:
Precision medicine is a groundbreaking approach that considers individual differences in genes, environments, and lifestyles for personalized healthcare. The Precision Medicine Initiative (PMI), launched by the National Institutes of Health (NIH), aims to understand how these factors impact disease prevention and treatment. The initiative’s goals include short-term cancer research advancements and long-term integration of precision medicine across healthcare. The “All of Us” research program involves over one million volunteers contributing health data to predict disease risks and improve diagnosis and treatment (National Institutes of Health (NIH)) (MedlinePlus) (National Institutes of Health (NIH)).
Placebo Effect:
The placebo effect, often seen in clinical trials, is a phenomenon where patients experience improvements in their condition despite receiving a non-active treatment. This effect is influenced by various factors, including patient expectations and the doctor-patient relationship. Harvard Medical School emphasizes that understanding the placebo effect can enhance the efficacy of treatments, as it highlights the power of the mind-body connection in healing (National Institutes of Health (NIH)).
Ethical Considerations and Future Directions
While placebo innovations offer exciting opportunities for enhancing clinical research and patient care, they also raise important ethical questions. For example, how can we ensure that patients are fully informed about the use of placebo interventions in clinical practice? How can we balance the potential benefits of placebo effects with the need for transparency and informed consent?
These are complex issues that require ongoing dialogue and collaboration among researchers, healthcare providers, policymakers, and patient advocates. As we continue to advance our understanding of placebo science, it will be important to develop ethical frameworks and guidelines that prioritize patient autonomy, beneficence, and justice.
Looking forward, there are many exciting avenues for future research on placebo innovations. For example, researchers are exploring the potential of digital health technologies, such as mobile apps and wearable devices, to enhance placebo effects and personalize treatment strategies. Other promising areas of research include the use of placebo interventions in the treatment of chronic pain, depression, and other complex medical conditions.
| Ethical Considerations | Future Directions |
|---|---|
| Ensuring patient informed consent | Exploring digital health technologies for enhancing placebo effects |
| Balancing benefits with transparency | Personalizing placebo treatments through innovative approaches |
| Prioritizing patient autonomy | Investigating placebo interventions in treating complex medical conditions |
| Promoting beneficence and justice | Developing ethical frameworks and guidelines for placebo use |
| Fostering ongoing dialogue and collaboration | Enhancing patient education on the role of placebos in clinical practice |
The Strengthening Placebo Effect
- Placebo response rates have increased over time, affecting clinical trials and treatment outcomes
- Multiple factors contribute to this phenomenon, including patient expectations and cultural shifts
- Researchers and healthcare professionals must adapt to the strengthening placebo effect
Evidence of Increasing Placebo Response Rates
Over the past few decades, researchers have observed a curious trend: placebo response rates in clinical trials have been steadily increasing. A 2015 study published in the journal Pain analyzed clinical trials for neuropathic pain conducted between 1990 and 2013. The researchers found that placebo response rates increased significantly during this period, from an average of 18% in 1990 to 30% in 2013.
This trend is not limited to pain studies. A 2015 analysis of antidepressant trials conducted between 1980 and 2009 revealed a similar pattern. The study, published in the Journal of Clinical Psychiatry, found that placebo response rates increased from an average of 30% in the earliest trials to 45% in the most recent ones.
Potential Factors: Patient Expectations, Trial Designs, Cultural Shifts
Several factors may contribute to the strengthening placebo effect. One key factor is patient expectations. As public awareness of the placebo effect has grown, patients may be more likely to believe that they will benefit from treatment, even if they receive a placebo. This heightened expectation can lead to a stronger placebo response.
Changes in clinical trial design may also play a role. Modern trials often include more frequent and intensive assessments, which can increase patient-provider interactions and potentially enhance the placebo effect. Additionally, the use of more subjective outcome measures, such as patient-reported pain scores, may be more susceptible to placebo responses than objective measures like blood tests.
Cultural shifts in healthcare and society may also contribute to the strengthening placebo effect. The rise of patient-centered care and shared decision-making has empowered patients to take a more active role in their treatment. This increased engagement and sense of control may enhance the placebo response. Furthermore, the growing popularity of complementary and alternative medicine, which often emphasizes the mind-body connection, may prime patients to be more responsive to placebos.
Implications for Clinical Practice and Drug Development
The strengthening placebo effect has significant implications for both clinical practice and drug development. In clinical trials, a stronger placebo response can make it more difficult to demonstrate the efficacy of new treatments. This may lead to promising therapies being abandoned prematurely or require larger and more expensive trials to achieve statistical significance.
For healthcare professionals, the strengthening placebo effect underscores the importance of harnessing the power of patient expectations and the therapeutic relationship. By fostering a positive, empathetic, and collaborative environment, providers may be able to enhance the placebo response and improve treatment outcomes.
Strategies for Addressing the Strengthening Placebo Effect
Researchers and drug developers are exploring various strategies to account for the strengthening placebo effect in clinical trials. Some approaches include:
- Using active placebos that mimic the side effects of the active treatment, making it harder for patients to guess whether they are receiving the real drug
- Incorporating novel trial designs, such as sequential parallel comparison design (SPCD), which can help to minimize placebo responses
- Employing objective outcome measures, such as biomarkers or imaging techniques, that are less susceptible to placebo effects
- Conducting trials in populations with lower placebo response rates, such as treatment-resistant patients or those with more severe symptoms
By adapting to the changing landscape of the placebo effect, researchers and healthcare professionals can continue to develop and deliver effective treatments for patients in need.
Addressing Criticisms of Placebo Use
- Ethical concerns and strategies for informed consent
- Balancing patient autonomy with therapeutic benefits
- Examining the main arguments against placebo use in clinical practice
While the strengthening placebo effect offers exciting possibilities for enhancing treatment outcomes, the use of placebos in clinical practice is not without controversy. One of the main criticisms of placebo use revolves around the ethical concerns of deception and informed consent.
Ethical Concerns and Informed Consent
Traditionally, placebos have been administered to patients without their knowledge or consent, raising questions about the ethics of deceiving patients in the name of treatment. This deception can undermine trust in the doctor-patient relationship and violate the principle of patient autonomy.
However, researchers are exploring strategies for open-label and authorized placebo use, where patients are fully informed about receiving a placebo and still experience therapeutic benefits. A 2010 study by Kaptchuk et al. found that patients with irritable bowel syndrome who knowingly received placebos reported significant improvements in symptoms compared to a no-treatment control group.
Balancing Autonomy and Therapeutic Benefits
While informed consent is crucial, some argue that the potential therapeutic benefits of placebos may justify their use in certain situations. For example, when no effective treatment exists or when the risks of active medication outweigh the benefits, placebos could offer a safe and potentially beneficial alternative.
Balancing Patient Autonomy with Therapeutic Benefits: A Case Study
In navigating the ethical dilemma of placebo use, a real-world example can shed light on the complexities of balancing patient autonomy with therapeutic benefits. Consider a scenario where a patient with chronic pain has exhausted conventional treatment options and is experiencing severe side effects from long-term medication use. Despite the lack of evidence supporting the efficacy of placebo interventions, the patient expresses a strong desire to explore alternative therapies.
In this case, healthcare providers face a challenging decision. On one hand, honoring the principle of patient autonomy requires fully informing the patient about the uncertain nature of placebo treatments and obtaining their informed consent. However, there’s also a consideration for the potential therapeutic benefits of placebos in alleviating the patient’s suffering when no other viable options remain.
The ethical dilemma arises from the tension between respecting the patient’s right to make informed choices about their healthcare and the possibility of offering a treatment that may provide relief, albeit through mechanisms not fully understood or scientifically validated.
This case underscores the importance of engaging in thorough discussions with patients about the risks, benefits, and uncertainties associated with placebo interventions. It highlights the need for healthcare providers to navigate the delicate balance between respecting patient autonomy and promoting therapeutic outcomes, even in situations where evidence is limited or conflicting.
Arguments Against Placebo Use
Critics argue that the use of placebos in clinical trials is unethical because it denies some participants access to potentially effective treatments. Additionally, the reliance on placebos in research may slow the development of new, active interventions.
Another concern is that the widespread use of placebos could lead to a “dumbing down” of medical care, where doctors rely on the placebo effect rather than actively seeking the most effective treatments for their patients.
The Role of Patient Expectations
The controversy surrounding the placebo effect also stems from the role of patient expectations in treatment outcomes. Some argue that the placebo effect is nothing more than a psychological trick, relying on patient gullibility rather than any real physiological changes.
However, research has shown that the placebo effect can lead to measurable changes in brain activity and physiological processes, suggesting that it is more than just a psychological phenomenon.
The placebo effect is a fascinating phenomenon where patients experience real physiological changes even when receiving a non-active treatment. Numerous studies have shown that the placebo effect can influence brain activity and hormone levels.
For example, research has demonstrated that placebo treatments can activate specific brain regions associated with pain relief, such as the prefrontal cortex and the anterior cingulate cortex. These brain areas are involved in emotional regulation and pain processing, which contributes to the observed analgesic effects of placebos. Additionally, placebos have been shown to increase the release of endogenous opioids, which are the body’s natural painkillers, further influencing pain perception (National Institutes of Health (NIH)).
In another study, placebo treatments were found to impact hormone levels. For instance, in patients with Parkinson’s disease, receiving a placebo led to increased dopamine release in the brain. This was observed using brain imaging techniques, indicating that placebos can trigger physiological changes similar to those produced by active medications.
These examples highlight the mind-body connection and illustrate how beliefs and expectations can induce tangible physiological effects. Understanding the placebo effect is crucial for enhancing treatment outcomes and underscores the importance of psychological factors in healthcare.
As the debate continues, it is clear that the use of placebos in clinical practice requires careful consideration of ethical principles, patient autonomy, and the potential for therapeutic benefits. By exploring strategies for open-label placebo use and prioritizing informed consent, healthcare providers can work to harness the power of the placebo effect while respecting patient rights and autonomy.
The Continuing Role of Placebos
- Placebos remain a vital tool in medical research and clinical trials
- Growing interest in harnessing placebo effects to enhance patient care
- Understanding placebo mechanisms key to optimizing treatment outcomes
Despite the controversy surrounding their use, placebos continue to play a crucial role in modern medicine. In randomized controlled trials (RCTs), the gold standard for evaluating treatment efficacy, placebos serve as a control to help distinguish the specific effects of an intervention from non-specific factors like expectation and context.
Placebos in Clinical Trials: Ensuring Scientific Rigor
Placebos help researchers account for the powerful influence of patient expectations and the therapeutic context on treatment outcomes. By comparing an active treatment to a placebo control, researchers can more confidently attribute observed effects to the specific intervention rather than placebo factors.
The Ethics of Placebo Use in Trials
The use of placebos in clinical trials does raise ethical concerns, particularly when an effective treatment is available. However, placebos are generally only used when there is genuine uncertainty about the efficacy of a new treatment compared to existing options (a state of “clinical equipoise”). Rigorous ethical guidelines, including informed consent and provisions for minimizing patient risk, help ensure that placebo use in trials is justified and responsible.
Harnessing Placebo Effects in Clinical Care
Beyond their role in research, there is growing interest in leveraging placebo effects to enhance patient care. While deceptive use of placebos is generally considered unethical, some argue that the transparent use of non-deceptive or “open-label” placebos could offer benefits without undermining patient autonomy.
Studies have shown that even when patients know they are receiving a placebo, they can still experience improvement in symptoms for conditions like irritable bowel syndrome, chronic pain, and depression. These findings suggest that the ritual of treatment and the patient-provider relationship itself can elicit therapeutic effects.
Ethical and Legal Considerations
The use of placebos in clinical practice does raise ethical and legal questions. While the American Medical Association considers the deceptive use of placebos unethical, it acknowledges that the transparent use of placebos may be appropriate in some circumstances.
The American Medical Association (AMA) has specific ethical guidelines regarding the use of placebos in clinical practice. According to the AMA, a placebo is a substance administered to a patient that has no specific pharmacological effect on the condition being treated. The AMA states that the use of placebos should be consistent with good medical care. It emphasizes that while placebos can be effective, they should be used cautiously and ethically.
The AMA suggests that physicians should enlist the patient’s cooperation and explain that using a placebo could help better understand their medical condition. Physicians should also obtain general consent to administer a placebo, thereby respecting patient autonomy and fostering trust. The AMA strongly discourages the use of placebos solely to pacify difficult patients, as this places the physician’s convenience above the patient’s welfare. Instead, the AMA encourages the use of reassurance and encouragement to build trust and promote health outcomes (AMA Code Medical Ethics) (AMA Ethics Journal).
In the realm of research, placebos are often used as control treatments, although their use remains a topic of ethical debate. The AMA suggests that using placebos in research requires careful ethical consideration, particularly when it involves withholding standard treatments from patients in the control group (AMA Ethics Journal).
The legality of placebo prescribing varies by jurisdiction. In the US, there is no federal law specifically regulating the clinical use of placebos, but physicians must still adhere to general principles of informed consent and avoid deceptive practices.
Understanding Placebo Mechanisms
Placebo research has also shed light on the complex interplay of biological, psychological, and contextual factors that shape healing processes. Advances in neuroscience have revealed how placebos can modulate pain perception, immune function, and even brain activity in ways that promote recovery.
For example, studies using functional brain imaging have shown that placebos can activate the brain’s endogenous opioid system, mimicking the effects of actual pain medications. This suggests that placebo effects are not just subjective reporting bias, but involve measurable physiological changes.
By elucidating these “non-specific” mechanisms of healing, placebo research may point the way to optimizing healthcare delivery and the patient experience to maximize therapeutic outcomes. This includes attention to factors like the quality of the patient-provider relationship, the therapeutic ritual and context, and patient mindset and expectations.
Placebo-Induced Changes in Brain Activity During Pain Perception: A Functional MRI Study
In this study, conducted by Wager et al. (2004), researchers investigated the neurobiological mechanisms underlying the placebo effect on pain perception using functional magnetic resonance imaging (fMRI). Participants with chronic pain conditions were subjected to painful stimuli while undergoing fMRI scans. They were informed that they would receive either an analgesic medication or a placebo. However, unbeknownst to the participants, they all received a placebo. The fMRI scans revealed significant changes in brain activity in regions associated with pain perception, such as the anterior cingulate cortex and the insular cortex, when participants believed they were receiving an analgesic compared to when they believed they were receiving a placebo. Interestingly, these changes in brain activity correlated with reported reductions in pain intensity, highlighting the intricate relationship between cognitive expectations, brain activity, and subjective experiences of pain. This study provides concrete neuroscientific evidence of how placebo interventions can modulate brain processing of pain perception, shedding light on the underlying neural mechanisms of the placebo effect in the context of pain management.
Harnessing Placebo Innovations for Better Patient Care
- Placebo innovations offer new strategies to optimize treatment plans and outcomes
- Open discussions about placebo effects can empower patients and enhance care
- Collaborating with patients as partners is key to maximizing placebo benefits
Recent advancements in understanding the placebo effect have opened up new possibilities for improving patient care. By integrating placebo-optimizing strategies into treatment plans, healthcare providers can harness the power of the mind-body connection to enhance healing and well-being.
Integrating Placebo-Optimizing Strategies into Treatment Plans
One key way to harness placebo innovations is by thoughtfully integrating placebo-optimizing strategies into patient treatment plans. This involves considering factors such as:
The Power of Positive Expectations
Setting positive expectations can significantly influence treatment outcomes. Healthcare providers can work with patients to cultivate an optimistic mindset, emphasizing the potential benefits of their treatment plan. This may involve:
- Clearly explaining the expected positive outcomes of the treatment
- Sharing success stories of other patients who have undergone similar treatments
- Encouraging patients to visualize their healing process and desired results
The Role of Ritual and Symbolism
The ritual and symbolism surrounding a treatment can also enhance placebo effects. Providers can thoughtfully design the treatment experience to incorporate elements such as:
- A calming, supportive environment
- Meaningful interactions and attentive care from healthcare staff
- The use of positive imagery or symbols associated with healing
By carefully crafting the treatment context, providers can tap into the power of placebo effects to support patient healing.
Open-Label Placebo for Chronic Pain Management: A Case Study
In this case study published in the Journal of Medical Ethics, a healthcare provider implemented an open-label placebo (OLP) intervention for managing chronic pain in a patient with fibromyalgia. The patient had previously tried various conventional treatments with limited success and was experiencing significant pain and reduced quality of life. The healthcare provider explained the concept of placebos honestly to the patient and discussed how the placebo effect could potentially benefit them.
The patient consented to try the OLP intervention as part of their treatment plan. Over the course of several weeks, the patient reported noticeable improvements in pain levels, mood, and overall well-being. The integration of OLP alongside other treatments in the patient’s care plan exemplifies how healthcare providers can ethically harness the placebo effect to enhance patient outcomes in chronic pain management.
Mind-Body Therapies and Placebo Effects in Anxiety Disorders: A Clinical Case Example
This case study, featured in the Journal of Anxiety Disorders, highlights the integration of mind-body therapies and placebo effects in the treatment of anxiety disorders. A healthcare provider worked with a patient diagnosed with generalized anxiety disorder (GAD) who was experiencing significant distress and impairment in daily functioning. Alongside evidence-based treatments such as cognitive-behavioral therapy (CBT) and medication, the provider incorporated mind-body interventions known to elicit placebo responses, such as mindfulness meditation and relaxation techniques, into the patient’s treatment plan. The patient responded positively to the integrated approach, reporting reductions in anxiety symptoms, improved coping skills, and a greater sense of control over their condition. This case underscores the potential synergy between evidence-based therapies and placebo-optimizing strategies in enhancing treatment outcomes for anxiety disorders.
Empowering Patients Through Open Discussion of Placebo Effects
Another important aspect of harnessing placebo innovations is openly discussing placebo effects with patients. Rather than viewing placebo effects as something to be minimized or controlled for, providers can empower patients by educating them about the potential benefits.
This open dialogue may involve:
- Explaining the science behind placebo effects and how they can influence healing
- Discussing the role of the mind-body connection in health and well-being
- Encouraging patients to take an active role in their own care by harnessing the power of positive expectations
By bringing placebo effects out into the open, providers can help patients feel more informed and engaged in their treatment process. This sense of empowerment can, in turn, enhance the very placebo effects that support healing.
Collaborating with Patients as Partners in Placebo-Enhanced Care
Finally, to fully harness the potential of placebo innovations, healthcare providers must view patients as active partners in their own care. This means moving beyond a paternalistic model of care to one that values patient input, preferences, and experiences.
Collaborating with patients may involve:
- Actively seeking patient perspectives and incorporating their goals into treatment plans
- Providing opportunities for patients to make informed choices about their care
- Encouraging patients to take an active role in their healing through practices like visualization, mindfulness, and self-care
By working together as partners, providers and patients can co-create a care experience that optimizes placebo effects and supports holistic healing.
As our understanding of placebo effects continues to evolve, healthcare providers have a powerful opportunity to harness these innovations for better patient care. By integrating placebo-optimizing strategies, openly discussing placebo effects, and collaborating with patients as partners, we can tap into the mind-body connection to enhance healing and well-being for all.
Collaborating with patients around placebo effects presents both challenges and ethical considerations that healthcare providers must navigate. Here are some of the main challenges and ways to address them:
1. Informed Consent and Honesty
- Challenge: Maintaining transparency and honesty while discussing the placebo effect with patients can be challenging. Some patients may feel deceived or distrustful if they perceive that their provider is offering a treatment with no active ingredient.
- Addressing the Challenge: Healthcare providers should prioritize informed consent and honesty when discussing placebo effects with patients. This involves explaining the concept of placebos in clear, understandable terms and discussing the potential benefits and limitations openly. Emphasizing that the placebo effect is a real phenomenon that can complement other treatments may help build trust and alleviate concerns.
2. Patient Expectations and Beliefs
- Challenge: Patient expectations and beliefs about treatment can influence the magnitude of the placebo response. Managing these expectations effectively is essential for optimizing treatment outcomes.
- Addressing the Challenge: Healthcare providers should engage in open and collaborative discussions with patients to understand their beliefs, expectations, and treatment preferences. By actively involving patients in decision-making and tailoring treatment plans to align with their beliefs and values, providers can enhance the placebo response and improve overall patient satisfaction.
3. Avoiding Harm and Maximizing Benefit
- Challenge: There is a risk of causing harm if patients forego evidence-based treatments in favor of placebos or if placebo interventions are not implemented responsibly.
- Addressing the Challenge: Healthcare providers must prioritize patient safety and well-being when considering placebo interventions. This involves ensuring that placebo treatments are used as adjuncts to, rather than replacements for, evidence-based therapies. Providers should also monitor patients closely for any adverse effects and adjust treatment plans accordingly. Additionally, integrating placebo-optimizing strategies, such as positive patient-provider interactions and contextual factors, can help maximize the therapeutic benefit of treatments while minimizing potential harm.
4. Cultural and Ethical Considerations
- Challenge: Cultural beliefs and ethical norms regarding placebo use may vary among different populations, leading to potential misunderstandings or conflicts.
- Addressing the Challenge: Healthcare providers should be sensitive to cultural differences and ethical considerations when discussing placebo effects with patients. Tailoring communication strategies and treatment approaches to respect patients’ cultural backgrounds and ethical values can help foster trust and collaboration. Additionally, ongoing education and training for healthcare providers on culturally competent care and ethical guidelines for placebo use can promote ethical practice and enhance patient-provider relationships.
By addressing these challenges and ethical considerations with sensitivity and care, healthcare providers can collaborate effectively with patients around placebo effects to optimize treatment outcomes while upholding ethical principles and promoting patient well-being.
The Future of Placebos: Personalized, Digitized, and AI-Optimized
The placebo effect is not just a scientific curiosity but a powerful tool in modern medicine. As researchers unravel the complexities of placebo responses, they are developing innovative strategies to harness their potential. From digital interventions and adaptive trial designs to biomarker-guided personalization and AI predictions, the future of placebos is looking brighter than ever.
These advancements promise to enhance clinical trial efficiency, personalize treatments, and deepen our understanding of the mind-body connection. By integrating placebo-optimizing approaches into patient care, healthcare providers can empower individuals to become active partners in their own healing process.
As you reflect on the transformative potential of placebo innovations, consider how you can contribute to this exciting field. Whether you are a researcher, healthcare professional, or simply someone fascinated by the power of the human mind, there are countless opportunities to make a difference.
What role do you see placebos playing in the future of medicine? How can we work together to unlock the full potential of placebo effects while upholding the highest ethical standards?
The placebo effect has stood the test of time, and with the latest advancements, it is poised to revolutionize healthcare as we know it. Let us embrace this opportunity to harness the power of the mind and create a future where personalized, placebo-enhanced care is accessible to all.